By Sayer Ji
Contributing Writer for Wake Up World
It has been known for some time that during pregnancy, the placenta enables a two-way trafficking of immune cells between mother and fetus. Some of the exchanged cells are capable of establishing long-lasting cell lines that persist for at least half a century after birth and are immunologically active.
Known as microchimerism, two genetically distinct and separately derived populations of cells are present in the same individual or organ. While it can occur artificially, as with recipients of blood transfusions or bone marrow transplants, it occurs naturally during most pregnancies. For instance, approximately 50-75% of women after giving birth carry fetal immune cells, [1] and about half as many offspring carry maternal immune cells.
The bidirectional fetomaternal trafficking of cells through the placenta is known as “fetal microchimerism” when moving in the fetal -> maternal direction, and “maternal microchimerism” when moving in the maternal -> fetal direction.
[pro_ad_display_adzone id=”110028″]
While much of the focus on this phenomenon in the past 15 years has been on the possible pathological role that these fetal cells play in contributing to autoimmune diseases in the mother, i.e. the “bad microchimerism” proposed by Nelson JL in 1996, [2] a more positive perspective is beginning to emerge, also known as the “good microchimerism” hypothesis, which “…suggests that persistent fetal cells, instead of inducing a maternal immune response, provide a rejuvenating source of fetal progenitor cells that may have the capacity to participate in maternal tissue repair processes.” [3]
Published in the Journal of the American Medical Association in 2004 and titled “Transfer of fetal cells with multilineage potential to maternal tissue,” researchers discussed the role that fetal microchimerism may play in responding to maternal injury by developing multilineage capacity in maternal organs. They found evidence that male fetal microchimeric cells had differentiated into maternal epithelial tissues (thyroid, cervix, intestine and gallbladder), indicating their stem-cell like qualities and the possibility that they were contributing to repair and/or regeneration of those injured maternal tissues. [4]
More recently (2012), the journal Circulation Research published a remarkable article titled “Fetal cells traffic to injured maternal myocardium and undergo cardiac differentiation,” which investigated the possible explanatory role that fetal microchimerism has in the clinical observation of the high rate of recovery from heart failure in peripartum (occurring during the last month of gestation or the first few months after delivery) cardiomyopathy patients. [5] Their stated objective was to “…determine whether fetal cells can migrate to the maternal heart and differentiate to cardiac cells.” The researchers reported the following results:
“We report that fetal cells selectively home to injured maternal hearts and undergo differentiation into diverse cardiac lineages. Using enhanced green fluorescent protein (eGFP)-tagged fetuses, we demonstrate engraftment of multipotent fetal cells in injury zones of maternal hearts. In vivo, eGFP+ fetal cells form endothelial cells, smooth muscle cells, and cardiomyocytes.”
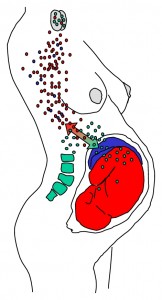 This astounding discovery indicates that fetal stem cells are capable of differentiating into a variety of heart cell types, including “beating cardiomyocytes,” and which may heal the mother’s physical heart.
This astounding discovery indicates that fetal stem cells are capable of differentiating into a variety of heart cell types, including “beating cardiomyocytes,” and which may heal the mother’s physical heart.
It appears that Nature made it possible for the unborn offspring of mammals to save their mother’s lives by contributing stem cells which are capable of grafting into tissues, including bone marrow, potentially providing a lifelong source of new healthy cells to replace damaged or dysfunctional ones.
In a future article we will be investigating the role of placental stem cells in explaining the phenomenon of placentaphagy, i.e. the near universal practice of mammals consuming the placenta of their young.
Article References
[1] Maternal microchimerism in healthy adults in lymphocytes, monocyte/macrophages and NK cells. Lab Invest. 2006 Nov ;86(11):1185-92. Epub 2006 Sep 11. PMID: 16969370
[2] Nelson JL (1996) Viewpoint. Maternal-fetal immunology and auto-immune disease: is some autoimmune disease auto-alloimmune or allo-autoimmune? Arthritis Rheum 39, 191–194 PMID: 8849367
[3] Fetal cells in maternal tissue following pregnancy: what are the consequences? Hum Reprod Update. 2004 Nov-Dec;10(6):497-502. Epub 2004 Aug 19. PMID: 15319378
[4] Transfer of fetal cells with multilineage potential to maternal tissue. JAMA. 2004 Jul 7 ;292(1):75-80. PMID: 15238593
[5] Fetal cells traffic to injured maternal myocardium and undergo cardiac differentiation. Circ Res. 2012 Jan 6 ;110(1):82-93. Epub 2011 Nov 14. PMID: 22082491
About the Author
Sayer Ji is the founder and director of GreenMedInfo.com and co-author of the book The Cancer Killers: The Cause Is The Cure with New York Times best-seller Dr. Ben Lerner and Dr. Charles Majors. His writings and research have been published in the Wellbeing Journal, the Journal of Gluten Sensitivity, and have been featured on Mercola.com, NaturalNews.com, Reuters.com, GaryNull.com, Infowars.com and Care2.com.
[pro_ad_display_adzone id=”110027″]







